
Business Support
As the leading outsourcing business in the industry, we have dedicated resources supporting our sales functions in driving clients’ growth strategies and maximising productivity.
Our support includes:
- Client management team
- Consumer brand management team
- Finance and reporting team
- Logistics management team
- Pharmacoviligance expert, accredited by MHRA
- Customer service team

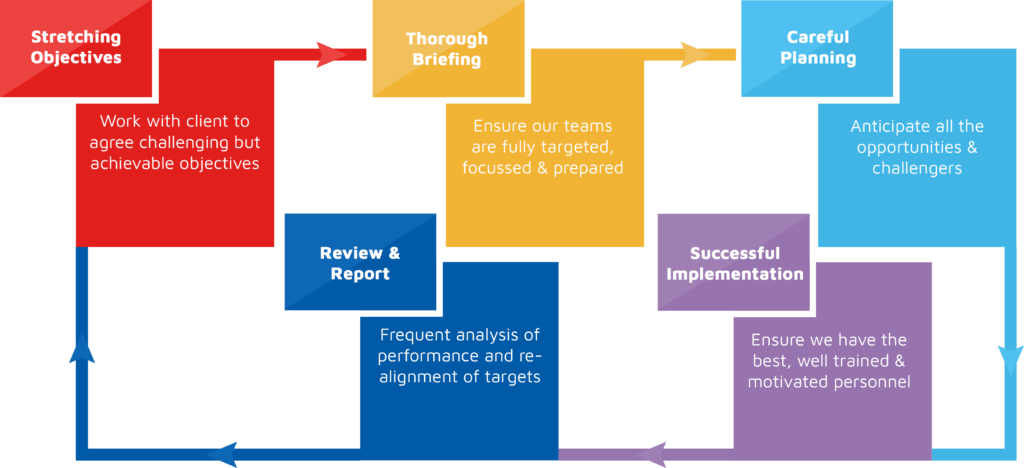
Managing Retailer and Client Expectations
Our established framework sets the standard for long term commercial relationships
with both clients and retailers:
What can we do for your brands?
Our outsourcing solutions can help your brands grow across channels and
throughout global markets.
Contact Us
Please use the details below to contact us or submit a message and we will get back to you shortly.
41 Richmond Hill, Bournemouth, BH2 6HS, UK
0344 243 6661
+44 1202 780 558




